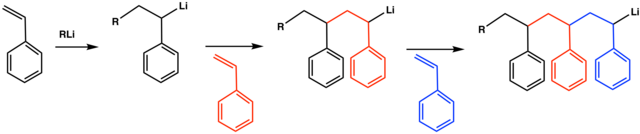
This type of polymerization is often employed to produce synthetic polydiene rubbers solution styrene butadiene rubbers sbr and thermoplastic styrenic elastomers.
Anionic vinyl polymerization.
That is an ion with a negative electrical charge.
That is small molecules containing carbon carbon double bonds.
They make up largest family of polymers.
Cationic polymerization of polar monomers such as vinyl ethers and styrene derivatives played a critical role in accomplishing ideal living cationic polymerization of vinyl type monomers.
Let s see how we get from a vinyl monomer to a vinyl polymer using for an example the simplest vinyl polymer polyethylene polyethylene is made from the monomer ethylene which is also called ethene when polymerized the ethylene molecules are joined along the axes of their.
The type of reaction has many manifestations but traditionally vinyl monomers are used.
131 the polymerization proceeds by formation of carbanions at polymer chain ends.
This type of polymerization is mostly used for the production of synthetic rubbers styrene solutions etc.
Anionic polymerization involves the polymerization of vinyl monomers possessing strong electronegative groups.
Anionic addition polymerization is a form of chain growth polymerization or addition polymerization that involves the polymerization of monomers initiated with anions.
Anionic ring opening copolymerization of vfcge and ethylene oxide eo generates stimuli responsive multifunctional poly viny.
The first orthogonal ferrocene monomer vinyl ferrocenyl glycidyl ether vfcge for both anionic and radical polymerization without the need of a protection group is presented.
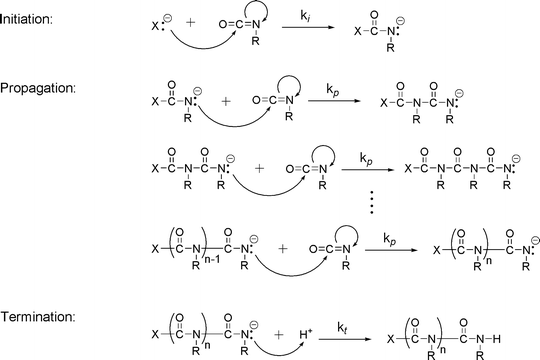
Anionic polymerizations can be divided into initiation propagation and termination chain transfer does not occur to any appreciable extent and thus the polymerization may be living.
Alkenes having alkoxy phenyl vinyl and 1 1 dialkyl substituents are some examples of monomers used in the cationic polymerization.
It is a type of vinyl polymerization in anionic polymerization the process is begun by an initiator in this case the initiator is an anion.
Anionic polymerization is a form of chain growth polymerization that encompasses the polymerization of vinyl monomers with strong electronegative groups.
Each reactive monomer goes on to react with other monomers to form a linear polymer.
Thus in this chapter general aspects of cationic polymerization are discussed followed by a brief overview of early development of living cationic polymerization.
When a type of chain growth polymerization encompasses the vinyl monomers in polymerization by using electronegative groups then such a process is called anionic polymerization.
Anionic polymerization is a type of chain growth polymerization in which an anionic initiator transfers a charge to a vinyl monomer which then becomes reactive.